
#VENTANA ISCAN SOFTWARE#
PathLogix Anatomic Pathology software offers fast, efficient and cost-effective patient data reporting and storage for pathologists and their staffs. Our customers tell us that their labs now work smarter, with far fewer mistakes, thanks to the Brother slide label printing solution," says Jerry Grayson, Customer Support Manager, PathLogix Corporation. Adding the capability to our Surgical Pathology software to print professional-looking, bar-coded slide labels at an affordable price has gotten us new customers as well as made our existing customers very happy. "We needed to provide our customers with an efficient slide label printing solution that wouldn't take a bite out of their wallets, and Brother came through.
#VENTANA ISCAN SERIES#
P-touch Editor is included with every Brother QL Series label printer.ĭevelopers can enhance their laboratory applications by integrating this cost-effective, labeling printing solution directly into their software using Brother's software development tools. Labels can be printed from a number of applications, including Brother P-touch Editor labeling software, PathLogix Anatomic Pathology software, MS Word, Excel, and Access and more. Healthcare professionals can now print highly legible, customized 23mm laboratory slide labels, and a wide variety of other label sizes, accurately and efficiently in seconds from the Brother QL Series label printers†. The Brother QL Series label printers can help to reduce the mistakes that occur when handwritten labels are misread," says John Biancamano, Brother Product Manager. This is an inefficient, unprofessional, and oftentimes illegible way to communicate extremely important information. Other printing solutions are expensive, and many lab technicians still hand write information on the slide labels. "In working with PathLogix Corporation, we learned that there was a need for a legible, cost-effective way to label laboratory slides.
#VENTANA ISCAN PROFESSIONAL#
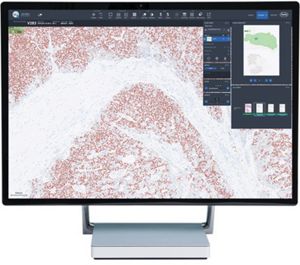
In response to this issue, Brother International Corporation, the leading manufacturer of professional labeling systems, has introduced an efficient and cost-effective laboratory slide label printing solution, which includes their new 23mm, DK-1221 slide labels. Accurately communicating these test results is critical for the medical decisions that affect patient care. medical laboratories, affecting more than two-thirds of all medical decisions. Boston Scientific won’t buy majority stake in M.I.Bridgewater, NJ - According to published work from the Centers for Disease Control and Prevention (Boone, 2003), billions of tests are performed each year in U.S.FDA approves Zoll therapy for sleep apnea with MRI.The top cardiac care innovation news at Heart Rhythm 2023.Analysts still high on Insulet despite Medtronic’s EOFlow buy, stock market reaction.The Tuscon, Ariz.-based company was acquired by Roche in 2008 for $3.4 billion.įiled Under: Imaging, News Well, Regulatory/Compliance Tagged With: Ventana Medical Systems "It represents another significant step by Ventana in assisting pathologists with the consistent and objective interpretation of these important breast cancer biomarkers, supporting the highest standards of patient care." “This most recent addition of the Ventana ER (SP1) Companion Algorithm software to our digital pathology portfolio demonstrates our continued commitment to provide our customers with the most advanced, clinically validated, pathology solutions available," digital pathology and workflow VP Dr. Ventana is now the only company in the industry "offering a comprehensive portfolio of FDA-cleared image analysis algorithms and digital read applications for the five key immunohistochemistry breast markers," according to a press release. The software has been cleared for 2 uses: clinical use, to semi-quantify the ER biomarker and digital use, to manually read and score the ER biomarker in lieu of a microscope. The software is used in conjunction with the Ventana iScan Coreo scanner.

Ventana Medical Systems, a Roche (PINK: RHHBY) subsidiary, landed 510(k) clearance from the FDA for the Companion Algorithm image analysis software.


 0 kommentar(er)
0 kommentar(er)
